Dark septate endophytes (DSE) are a heterogenous group of conidial or sterile fungi (thought to be ascomycetes) with darkly-pigmented, septate hyphae that commonly colonize plant roots. Most DSE also produce intracellular structures called microsclerotia. It has been suggested that these microsclerotia may serve as dispersal structures (Currah et al., 1993), although the actual purpose appears to be unknown. Perhaps they serve a storage function for the fungi as do the vesicles of AM fungi?
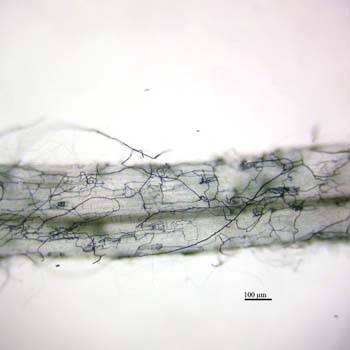
The following images show septate hyphae of a DSE growing in and around a root of Eurybia divaricata. The first image shows net-like strands formed by the hyphae of a DSE:
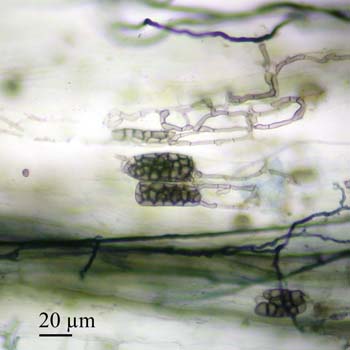
The next image depicts several hyphae ending in microsclerotia, which appear as grape-like clusters.
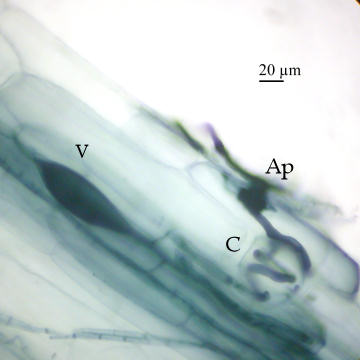
Another morphotype can be seen with a root of Maianthemum racemosum in the following image:

The taxonomy of this group is poorly understood and little is known of the role that DSE play in natural ecosystems (see review by Jumpponen and Trappe 1998). DSE are difficult to identify from field samples so many may not yet be known. A few of the species identified to date (mostly from bioassays) include Chloridium paucisporum, Leptodontidium orchidicola, Phialocephala dimorphosphora, Phialocephala fortinii, and Phialophora finlandia. Of these, Phialocephala fortinii appears to be the best studied. Unlike AM fungi, the DSE that have been studied to date do not require a plant host and many can be grown as pure cultures.
Available evidence suggests that DSE range from strongly pathogenic to non-pathogenic to mycorrhizal for plants. Some strains of DSE may be involved in host plant nutrient acquisition and it has been proposed that this may be a mutualistic, mycorrhiza-like relationship (Jumpponen and Trappe 1998). These relationships do not appear to be host specific (Jumpponen and Trappe 1998), with some strains forming ectendomycorrhizas but also associating with other hosts in ways not completely understood (Jumpponen 2001).
Although their mycorrhizal status is uncertain (Jumpponen and Trappe 1998), DSE are commonly associated with the roots of herbaceous and woody plants from alpine, boreal and northern temperate ecosystems (Haselwandter and Read 1980, Holdenrieder and Sieber 1992; ODell et al. 1993; Ahlich and Sieber 1996). To date, carbohydrate flow from a host plant to a DSE has not yet been demonstrated as it has for AMF (Jumpponen 2001). Despite their ubiquity in terrestrial ecosystems, the role of DSE in plant nutrient acquisition and plant community assemblage remains largely unknown. Given the ubiquity of DSE in many terrestrial ecosystems and their possible mycorrhiza-like relationships with plants, studying this group of fungi could increase our understanding of plant community assemblage.
Literature Cited:
Ahlich, K. and T.N. Sieber. 1996. The profusion of dark septate endophytic fungi in non-ectomycorrhizal fine roots of forest trees and shrubs. New Phytologist 132:259-270.
Currah, R.S., Tsuneda, A., and Murakami, S. 1993. Morphology and ecology of Phialocephala fortinii in roots of Rhododendron brachycarpum. Canadian Journal of Botany 71:1639-1644.
Haselwandter, K. and D.J. Read. 1980. Fungal associations of roots of dominant and sub-dominant plants in high-alpine vegetation systems with special reference to mycorrhiza. Oecologia 4:557-62.
Holdenrieder, O. and T.N. Sieber. 1992. Fungal associations of serially washed healthy non-mycorrhizal roots of Picea abies. Mycological Research 96:151-156.
Jumpponen, A. and J.M. Trappe. 1998. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytologist 140:295-310.
Jumpponon, A. 2001. Dark septate endophytes are they mycorrhizal? Mycorrhiza 11:207-211.
O’Dell, T.E., H.B. Massicotte and J.M. Trappe. 1993. Root colonization of Lupinus latifolius Agardh. and Pinus contorta Dougl. By Phialocephala fortinii Wang & Wilcox. New Phytologist 124:93-100.